Company
Based near Bordeaux (FR), IMPLANET is an international company with a US subsidiary in Boston, funded by European institutional and private investors.

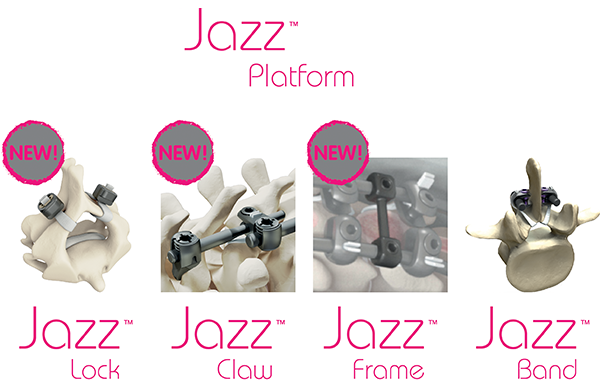
IMPLANET focuses on spine and knee products. Its flagship product, the Jazz™ latest generation implant, is intended to improve the treatment of spinal disorders requiring spinal fusion surgery. Protected by 4 sets of international patents, Jazz™ has obtained the 510(k) clearance from the Food and Drug Administration (FDA) in the United States and the CE mark.
Taking into account the evolutions in the healthcare system all over the world, the IMPLANET Marketing team identifies a portfolio of products and services based on a detailed analysis of socio-economic, regulatory, demographic and cultural specifications. The IMPLANET R&D team focuses on a scientifically and clinically proven implant design, using top quality materials tested to the highest prevailing standards, associated with simple and user-friendly instrumentations.
Our various workgroups, composed of surgeons, experienced engineers and product managers work closely together to offer a range of implants with scientifically sound specifications and highest quality to adequately respond to requirements of healthcare professionals.
Based on these market elements and facts, IMPLANET is able to provide the best of what science and experience have to offer, while ensuring to the stakeholders the best value for their products, their needs and demands.